The Unique Properties of Clay
MRSA, Methicillin Resistant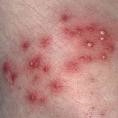 Staphylococcus Aureus, kills over 18,000
Americans every year. Other virulent pathogens include penicillin-resistant S.
aureus (PRSA), strains of Escherichia coli (E. coli), Salmonella, Buruli ulcers
(Mycobacterium ulcerans), another flesh-eating bacterium and relative of leprosy
(Bacillus Mycobacterium leprae) and tuberculosis (Mycobacterium tuberculosis)
that destroys the im
Staphylococcus Aureus, kills over 18,000
Americans every year. Other virulent pathogens include penicillin-resistant S.
aureus (PRSA), strains of Escherichia coli (E. coli), Salmonella, Buruli ulcers
(Mycobacterium ulcerans), another flesh-eating bacterium and relative of leprosy
(Bacillus Mycobacterium leprae) and tuberculosis (Mycobacterium tuberculosis)
that destroys the im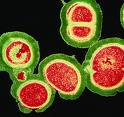 mune
systems, skin, tissues, and bones, causing death and disfigurement to children.
mune
systems, skin, tissues, and bones, causing death and disfigurement to children.
Because of its very special properties, clay can be used to kill all of the above and many other bacteria. What are the chemical and physical properties of clay?
First, clay is very alkaline. The pH of clay is between 8 and 10 depending on the type of clay.
Second, clay has a net negative charge. Things that attack our bodies, including the above bacteria, mercury, other heavy metals, toxins (from drugs and other sources), viruses, molds, yeasts and fungi, etc., tend to have a positive ionic charge.
In his book "Our Earth, Our Cure," Dr. Raymond Dextreit
describes clay as a "greasy sort of earth." The microscopic particles of clay
are short, wide, and flat, similar to a stack of credit cards. The flat surfaces
are negativel y
charged, and the narrow edges are positively charged. Because of the relatively
large surface area, a clay particle is extremely negatively charged.
y
charged, and the narrow edges are positively charged. Because of the relatively
large surface area, a clay particle is extremely negatively charged.
Dr. Robert T. Martin, Ph.D., Cornell University, and MIT mineralogist, stated that "one gram of clay has a surface area of 800 square meters or ... 10 football fields of negative pulling power." This powerful ionic negative "magnetic" force acts with unlimited ferocity toward all the positively charged ions listed above. The property of clay to adsorb and absorb positively charged ions, lock them in, and hold them until they can be excreted makes clay a very effective agent of detoxification.
Rossman Giese, Ph.D., professor of geology in University of Buffalo's College of Arts and Science used several techniques to study clays, including atomic force microscopy. "Unlike antibiotics, which are essentially chemical weapons against bacteria, antimicrobial clays kill through purely physical means," he explained. "The bacterium has to come into physical contact with the clay in order for something to happen. That contact turns deadly. The antimicrobial agents in the clay poke a hole in the cell wall of the bacterium, causing the bacterium to leak to death. There is no way that the bacterium can evolve to avoid it."
Dr. Lynda Williams, Ph.D., from Arizona State University, who tested the effect of clay on the most potent bacteria, said, "So far, clay has killed everything we tested."


